 On September 26, fecal egg counts ranged from 0 to 5680 and averaged 1584 epg. On September 10, the barber pole worm comprised 98% of the worm infection.
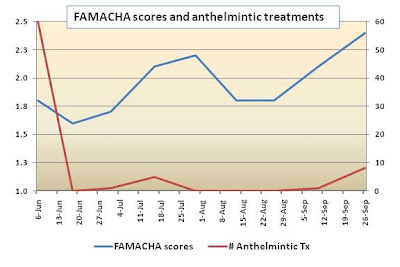
On September 26, fecal egg counts ranged from 0 to 5680 and averaged 1584 epg. On September 10, the barber pole worm comprised 98% of the worm infection.In previous years of the test, egg counts peaked earlier in the test (July or August). In addition, despite high egg counts towards the end of the test, few goats required deworming as indicated by FAMACHA© eye anemia scores. The need for deworming peaked on September 26 when 8 goats were dewormed. Of the 10 goats that await slaughter at the test site, four were dewormed on October 8.
The Western Maryland Pasture-Based Meat Goat Performance Test places heavy emphasis on internal parasite resistance and resilience. Goats that gain well, but have poor resistance or resilience data do not make the sale. Furthermore, just because a goat doesn't require deworming (has good FAMACHA© scores) doesn't mean he's suitable as a breeder. A breeding buck should also have low egg count data, so he and his progeny don't continuously contaminate the pastures with infective worm larvae.
 Parasite resistance was determined by fecal egg count data. Every two weeks a fecal sample was collected from the rectum of each goat. The samples were bagged, labeled, and stored on ice before being shipped overnite to Dr. Dahlia Jackson's parasitology lab at Delaware State University. Individual fecal egg counts were determined by the Modified McMaster procedure.
Parasite resistance was determined by fecal egg count data. Every two weeks a fecal sample was collected from the rectum of each goat. The samples were bagged, labeled, and stored on ice before being shipped overnite to Dr. Dahlia Jackson's parasitology lab at Delaware State University. Individual fecal egg counts were determined by the Modified McMaster procedure.An additional fecal sample was collected randomly from every third goat. The additional samples were combined into a single pooled sample. Samples from goats with poor FAMACHA© scores and/or loose stools were also favored for the pooled sample, as they would be more likely to have higher fecal egg counts. Fecal coproculture could not be performed on the sample from June 18, as the egg count was insufficient (only 33 epg).
 The pooled sample was not cooled. It was sent via overnite mail in a vacuum-sealed bag to the University of Georgia. Fecal coproculture (larvae ID) was done by Dr. Ray Kaplan's lab in the College of Veterinary Medicine. In addition to identifying parasite larvae, a pooled fecal egg count was determined.
The pooled sample was not cooled. It was sent via overnite mail in a vacuum-sealed bag to the University of Georgia. Fecal coproculture (larvae ID) was done by Dr. Ray Kaplan's lab in the College of Veterinary Medicine. In addition to identifying parasite larvae, a pooled fecal egg count was determined.Since the test ended on September 26, an additional set of individual fecal egg count data was received. Fecal coproculture data has been completed through September 10. One more set of data is expected.











